Cell Collection
Induced Pluripotent Stem Cell (iPSC) Lines
With over 500 unique iPSC lines spanning over 30 degenerative diseases, our bank of iPSC lines is available for most research purposes.
GMP iPSC Lines
Clinical-grade induced pluripotent stem cells (iPSCs) created under GMP (Good Manufacturing Practice) conditions are high-quality, clinical-grade cells designed for use in cell therapy, regenerative medicine, and clinical applications. These iPSCs are produced in compliance with rigorous standards to ensure safety, consistency, and reproducibility, making them ideal for clinical trials and therapeutic use. Matched Research Cell Banks (RCB) material is available for assessment and use in preclinical applications.

Gene Modified Cell Lines
Explore our collection of isogenic controls, disease knockout lines, and GFP/RFP expressing cell lines.
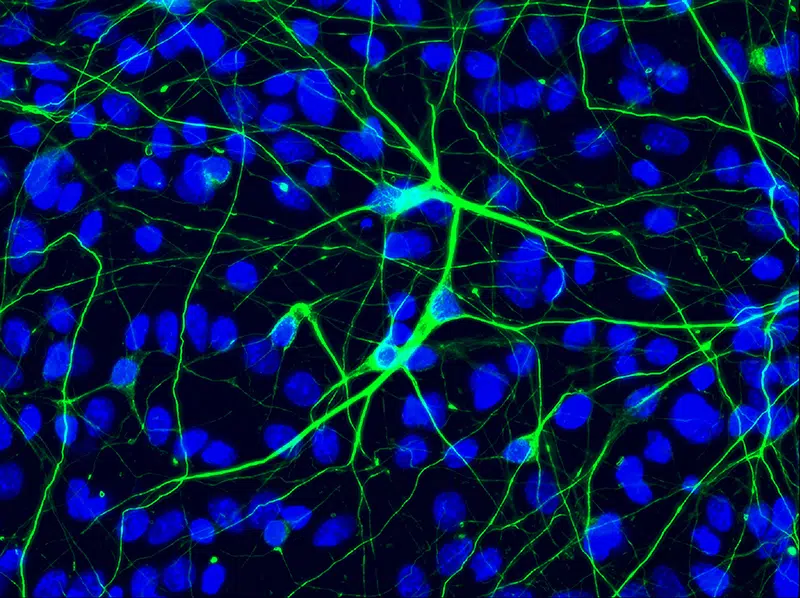
Neural Cells
A growing collection of neural precursor cells from both control and diseased cell lines.
Endothelial Cells
iPSC-derived vascular endothelial cells (iECs) provide an innovative approach to studying vascular biology. These cells mimic the properties of native human vascular cells while offering a renewable, scalable, and non-invasive source for research discovery and regenerative therapies. iECs enable personalized disease modeling, create a robust platform for disease modeling, drug discovery, toxicity screening, and promoting tissue repair. A growing collection of iECs available from healthy control donor iPSC lines is now available.
Embryonic Stem Cell (ESC) Lines
The CSES embryonic stem cell lines are part of the NIH Embryonic Stem Cell Registry.
